3 the Structure of 3-propylpentane Is Best Described as
Group of answer choices The central government should write both the US Constitution and state constitutions Disputes between states should always be settled by the national government Federal laws are more. Find the area of triangle.
2 Methyl 3 Ethylpentane C8h18 Chemspider
Economic growth is represented by.

. 3-Methyl-3-propylpentane c 111 -Trifluoro-2-methylpropane d 4-3-Bromobutylnonane. B Its chemical formula is C3H6. Which of the following structural formulas is an isomer to n-hexane.
Up to 25 cash back The structure of 3-propylpentane is best described as A. 3-propylpentane-24-dione Pre-Registration process. Choose all of the statements that would be TRUE for the molecule propane.
3-Propylpentane-24-dione C8H14O2 CID 73762 - structure chemical names physical and chemical properties classification patents literature biological. 3-Methyl-3-propylpentane-15-diol C9H20O2 CID 13928740 - structure chemical names physical and chemical properties classification patents literature. A five-carbon chain with a three-carbon branch on the third carbon in the chain.
A 362 cm c 257 cm. What is the longest carbon chain for the compound shown. A five-carbon ring with a three-carbon branch on the third carbon in the ring.
A five-carbon ring with a three-carbon branch on the third carbon in the ring. The reaction CH2CH2 Br2 CHBrCHBr would best be. The structure of 3-propylpentane is best described as a.
A three-carbon chain with a three-carbon branch on the third carbon in the chain b. C Draw Newman. Figure 311Refer to Figure 311.
B Carbon forms four bonds although the ground state configuration would predict the formation of fewer bonds. What makes carbon such a unique element. D It is the simplest hydrocarbon.
A five-carbon chain with a three-carbon branch on the third carbon in the chain. None of the above. D To a greater extent than any other element carbon can bond to.
The reaction CH2CH2 H2 CH3CH3 would best be described as. What is the most common type of bond found in organic molecules. Some substance identifiers may have been claimed confidential or may.
Other relevant information includes the following. For the methyl cation CH3 the geometry H-C-H bond angle and hybridization of the carbon atom is best described as. Round to the nearest whole number.
Someone who believed in states rights during the antebellum period would probably support which statement. They are saturated with hydrogen and only have single bonds between carbons. The approximate bond angles for the C-C-H bonds in the following molecules are.
Convert the following names into the corresponding molecular structures. Answers Biology 09012020 1231. C It is a gas at room temperature and standard pressure.
C Carbon forms covalent bonds rather than ionic bonds. The Substance identity section is calculated from substance identification information from all ECHA databases. Try placing the proton first on one and then.
26-dimethyl-3-heptyne C C 1 2. Some alpha-hydroxy acids are used in cosmetics and as an exfoliating agent to remove dead skin. Write an equation for the graph.
Write the structural formula for each of the following. Neither reflect other factors that affect the susceptibility of the effects described such as duration of exposure or substance concentration eg. A five-carbon chain with a three-carbon branch on the third carbon in the chain c.
In case of consumer and professional uses. Same molecular formula AND different structure. The structure of 3-propylpentane is best described as a.
A three-carbon chain with a five-carbon branch on the fifth carbon in the chain. The substance identifiers displayed in the InfoCard are the best available substance name EC number CAS number andor the molecular and structural formulas. A three-carbon chain with a three-carbon branch on the third carbon in the chain.
The structure of 3-propylpentane is best described as a. Isopropyl methyl bromide The structure below. Course Title CP 342.
What is the expected bond angle for the C-C-O bonds in the molecule below. What is the IUPAC name of the following A 6 isopropyl 3 methylnonane C 2 ethyl 5. What is the iupac name of the following a 6 isopropyl.
To the best of your ability assign relative energy values to all the conformations on your diagram. In this bond which of the following best describes electron density on the nitrogen atom. A five-carbon chain w.
A Elemental carbon comes in two forms diamond and graphite. Eight novel N-2-oxoindolin-3-ylidene-2-propylpentane hydrazide-hydrazone derivatives 4ah were synthesized and fully characterized by IR NMR 1 H-NMR and 13 C-NMR elemental analysis and. A three-carbon chain with a five-carbon branch on the fifth carbon in the chain.
A In complete combustion it yields three molecules of carbon dioxide. Trigonal planar 120 degsp2. After doing so check to see if the name of each molecule as given here is in.
School The College at Old Westbury. Suggest a structure for mathrmCH_3 mathrmCO_2 mathrmH_2. Minnesota State University Moorhead.
Several statements are given below. A three-carbon chain with a three-carbon branch on the third carbon in the chain b. Refer to the information provided in Figure 311 below to answer the question s that follow.

3 Ethyl 4 Propylheptane C12h26 Pubchem
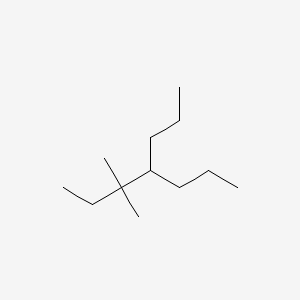
3 3 Dimethyl 4 Propylheptane C12h26 Pubchem
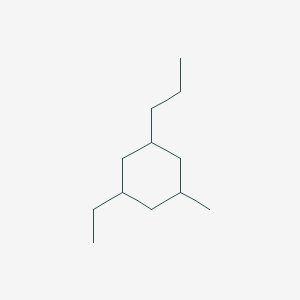
Cyclohexane 3 Ethyl 5 Methyl 1 Propyl C12h24 Pubchem

3 Methyl 4 Isopropylheptane C11h24 Pubchem

3 Ethyl 2 Propylpentane 1 1 4 Triol C10h22o3 Pubchem

4 Ethyl 3 5 Dimethyloctane C12h26 Pubchem

2s 3r 2 Methyl 3 Propylpentane 1 5 Diol Structure C9h20o2 Over 100 Million Chemical Compounds Mol Instincts
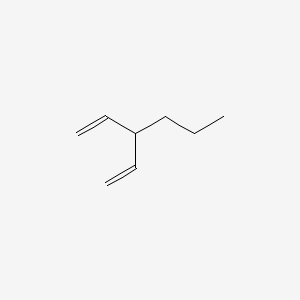
1 4 Pentadiene 3 Propyl C8h14 Pubchem
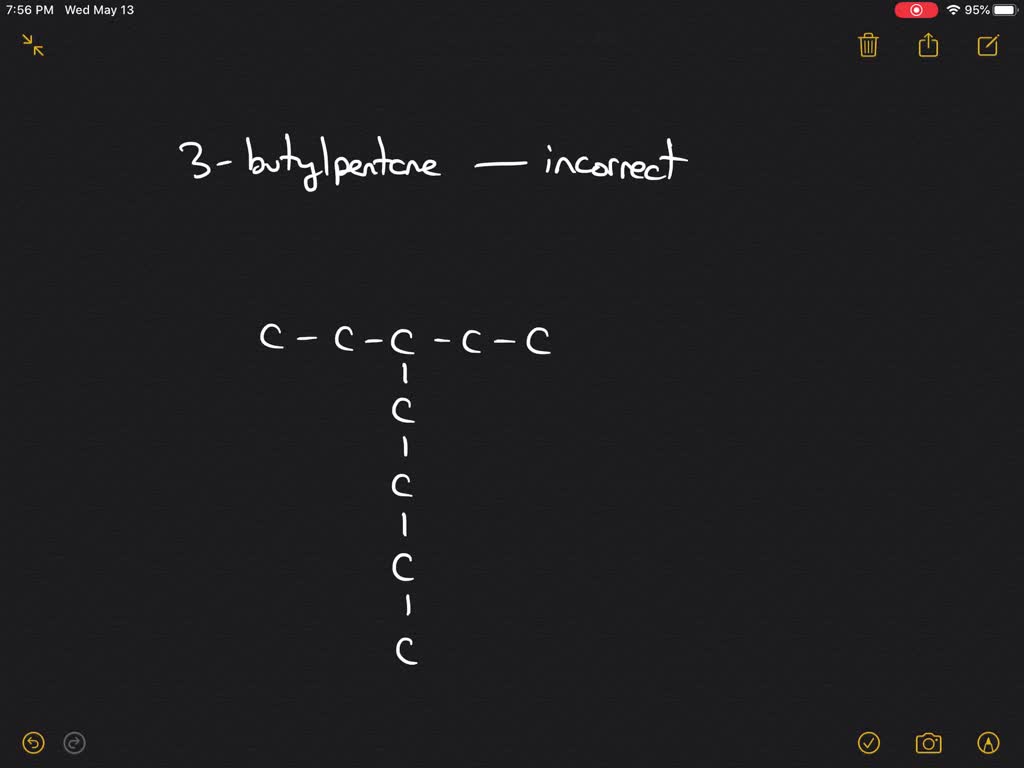
Solved Interpret Chemical Structures Why Is The Name 3 Butylpentane Incorrect Based On This Name Write The Structural Formula For The Compound What Is The Correct Iupac Name For 3 Butylpentane

Structure Of The 3 Propyl Pentane 2 4 Dione Download Scientific Diagram

2 3 4 Trimethylpentan 2 Ol C8h18o Pubchem

2 Hydroxy 3 Pentanone C5h10o2 Pubchem
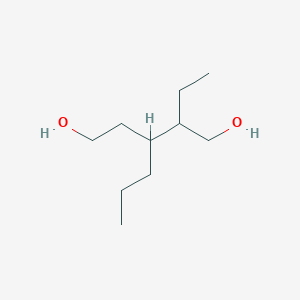
2 Ethyl 3 Propylpentane 1 5 Diol C10h22o2 Pubchem

3 Ethyl 3 Propylpentane 1 5 Diamine C10h24n2 Pubchem

4 Ethyl 3 Methyl 4 Propylheptane C13h28 Pubchem
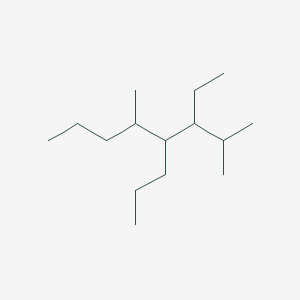
3 Ethyl 2 5 Dimethyl 4 Propyloctane C15h32 Pubchem

Structure Of The 3 Propyl Pentane 2 4 Dione Download Scientific Diagram
Comments
Post a Comment